1. Introduction
Cannabis sativa plant has been used for millennia for both medicinal and recreational purposes, but it is only in recent times that it drew the interest of modern medicine for its therapeutic and psychoactive properties (Merlin, 2003). An important advancement in cannabis research was the isolation and biochemical characterization of its two major components, the cannabinoids Δ9-tetrahydrocannabinol (Δ9-THC) (Gaoni & Mechoulam, 1971) and cannabidiol (CBD) (Adams et al., 1940), (Mechoulam & Shvo, 1963). This paved the way for the identification of the endocannabinoid system, which comprises cannabinoid receptors (CB1 and CB2), endogenous ligands (endocannabinoids), and the enzymes responsible for their synthesis and breakdown (Zou & Kumar, 2018). Cannabinoids exert their effects primarily by binding to cannabinoid receptors CB1 and CB2 or by influencing the signaling pathways of the endocannabinoid system (Joshi & Onaivi, 2019), which is involved in a wide range of physiological functions. These functions include pain regulation, immune function, appetite, and metabolism, as well as mood and stress regulation (Zou & Kumar, 2018).
Δ9-THC is the most abundant phytocannabinoid (cannabinoid derived from the cannabis plant) and is responsible for the majority of the cannabis plant’s psychoactive effects. These occur through the binding and activation of CB1 receptors, which are predominantly present in the central nervous system (Pertwee, 2008). CBD, although sharing with Δ9-THC a similar chemical structure, does not induce psychomimetic effects because of its lower affinity for the central CB1 receptors (Russo & Marcu, 2017).
Research on both rodents and humans has provided substantial evidence for the efficacy of CBD, either alone or in combination with Δ9-THC, in treating a plethora of different conditions, including epilepsy, nausea post-chemotherapy, and spasticity in multiple sclerosis (National Academies of Sciences, Engineering, and Medicine, 2017). Driven by the need for alternative and more effective treatments in psychiatry, interest has also grown in the potential use of cannabinoids for mental health (Joshi & Onaivi, 2021; Sarris et al., 2020). In 2023, more than 100 trials assessing the effects of CBD as a treatment for various psychiatric disorders were registered at clinicaltrials.gov. In fact, both animal and human studies have shown that CBD may have anxiolytic, antidepressant, and antipsychotic properties (Batalla et al., 2019; Iseger & Bossong, 2015; Rohleder et al., 2016). Converging evidence suggests the involvement of the endocannabinoid system in the etiology of several mental illnesses, such as depressive disorders (Hill & Gorzalka, 2005), anxiety disorders (Patel et al., 2014), psychotic disorders (Bossong & Niesink, 2010; Leweke & Koethe, 2008), and substance use disorders (Maldonado et al., 2006). As a result, the endocannabinoid system emerges as a highly promising target for intervention (Bright & Akirav, 2022; Sarris et al., 2020).
While most of the research on the application of cannabinoids in mental health has focused on Δ9-THC and CBD, to date about 120 other phytocannabinoids have been identified (ElSohly & Gul, 2014). These are often referred to as “minor cannabinoids” due to their less investigated biological profile (Caprioglio et al., 2022; Stone et al., 2020). Examples include cannabigerol (CBG), cannabichromene (CBC), cannabinol (CBN), Δ8-tetrahydrocannabinol (Δ8-THC), along with acidic cannabinoids such as Δ9-tetrahydrocannabinolic acid (Δ9-THCA) and cannabidiolic acid (CBDA), as well as propyl phytocannabinoids (varinoids) such as Δ9-tetrahydrocannabidivarin (Δ9-THCV) and cannabidivarin (CBDV) (Hanuš et al., 2016; Walsh et al., 2021).
A reason for these compounds being relatively overlooked is the challenge of isolating sufficient amounts (El-Alfy et al., 2010). However, recent cutting-edge preclinical research has made significant strides in characterizing their pharmacological profile and investigating their therapeutic potential (Stone et al., 2020; Walsh et al., 2021; Zagzoog et al., 2020). Some minor cannabinoids have already exhibited promising preclinical profiles, while being devoid of the psychomimetic effects of Δ9-THC (Walsh et al., 2021).
A better understanding of the behavioral pharmacology of the less studied components of cannabis is needed to validate their therapeutic potential and reject misleading information derived from subjective reports only. Some recent reviews have provided a general overview of the possible therapeutic uses of minor cannabinoids (Caprioglio et al., 2022; Stone et al., 2020; Walsh et al., 2021), whereas others have summarized the medicinal effects of cannabinoids (Black et al., 2019; McKee et al., 2021; Sarris et al., 2020), and CBD in particular (Batalla et al., 2019; García-Gutiérrez et al., 2020; Kwee et al., 2023), in neuropsychiatric disorders. To our knowledge, this is the first systematic review to critically assess preclinical and clinical studies that have investigated the therapeutic potential of minor cannabinoids in psychiatric disorders. With this work, we aim to shed light on which therapeutic applications are the most promising and which compounds are worth further investigation.
2. Material and methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 statement (Page et al., 2021), and it was registered on Open Science Framework (OSF) Registries on May 3, 2023.
2.1. Search strategy
Literature searches were performed up to April 3, 2023, using PubMed/MEDLINE, Scopus, EMBASE, and PsycINFO databases. No restrictions on language or year of publication were applied. In addition, the registers “animalstudyregistry.com” and “preclinicaltrials.eu” were consulted. The search was based on a combination of search terms referring to “minor cannabinoids” and “psychiatric disorders”. MeSH terms, keywords, text words, and search strings were used appropriately for each database. The full search strings for each database are provided in the Supplementary File (see S1). Reference sections of the included articles were also screened to identify additional relevant studies.
2.2. Inclusion and exclusion criteria
This systematic review has a broad scope, focusing on the effects of minor cannabinoids in both humans, and animal models of any psychiatric indication. The following inclusion and exclusion criteria were applied, in accordance with the PICO (Population, Intervention, Comparison and Outcome) framework.
Population type: Studies with humans and animal models of any psychiatric condition and any sample size were included. Not in-vivo studies were excluded.
Intervention type: Studies in which any minor cannabinoid was administered were included, regardless of the modality of administration and doses. Studies involving synthetic cannabinoids were also included if the abstract explicitly mentioned that they were analogues of minor cannabinoids with a similar biological profile. Studies exclusively investigating major cannabinoids (CBD and Δ9-THC), studies focusing on endocannabinoids, and studies involving synthetic compounds that act on the endocannabinoid system but are not specifically analogues of minor phytocannabinoids were excluded.
Comparison type: Studies comparing the effects of minor cannabinoids vs. placebo were included. Reviews, case reports, observational studies, and commentary articles were excluded.
Outcome type: Included studies explored the differences between the effects of the investigated cannabinoids and placebo conditions, with respect to the disease-related symptoms under study and/or the respective clinical or preclinical tests used for a specific condition. Studies reporting outcomes not related to psychiatric symptoms or conditions were also excluded.
2.3. Data collection
2.3.1. Data selection
Retrieved articles were imported into the web-based software tool for systematic reviews, Rayyan, where duplicates were removed. Two reviewers (GC and MV) independently screened the articles by title and abstract. Thereafter, the full texts of potentially relevant articles were reviewed for inclusion. Disagreements were resolved through discussion between the two reviewers. In cases where consensus could not be reached, other reviewers (LG and AB) were consulted.
2.3.2. Data extraction
Relevant data from the included articles was extracted by one of the two reviewers (GC or MV) and cross-checked by the other. The extracted data included the following: study information (title, name of the authors, year of publication); psychiatric condition under study; cannabinoid under study (with respective doses, administration route, and number of administrations); sample characteristics (sample size, sex, and, for preclinical studies, animal strain and housing); tests performed; and outcomes.
The outcomes included descriptive results based on the author’s conclusions (significant increase/decrease/no significant effect compared to the control group) and quantitative outcomes (mean effects and standard deviations of the experimental and control groups). Since most preclinical studies reported quantitative outcomes only graphically, mean effects and standard error measures for both experimental and control groups were extracted from figures by two reviewers (GC, MV) using a digital ruler (Universal Desktop Ruler). Subsequently, standard deviations were calculated based on mean effects, sample sizes, and standard error measures. When sample sizes were reported as a range, the lowest sample size was utilized for calculations. If upper and lower confidence limits were reported instead of the standard error measures, the limit representing the largest deviation from the mean was used to calculate the standard deviation. Tests with missing or unclear information regarding sample size and error measures were excluded from further data synthesis steps.
As some of the included studies assessed the effects of a compound using different tests with multiple outcomes, information to extract was selected upfront. Two experienced preclinical researchers (LG, MV) identified a primary test and primary outcome for each experimental group. These were defined as the most representative to explore the specific psychiatric disorder under study. Multiple tests assessing the same compound could be selected when different groups of animals were used. Thereafter, a psychiatrist and a resident in psychiatry (AB, PvdM) were consulted to confirm the translational relevance of the selected outcomes to human psychiatric disorders. If multiple doses of cannabinoids were tested, the dose yielding the largest effect was extracted.
2.4. Data synthesis
Due to the heterogeneity of the retrieved data regarding the conditions under study, the compounds examined, and the tests performed, conducting a meta-analysis was not feasible. Therefore, we opted for a qualitative data synthesis approach. This involved organizing and structuring the data separately based on the psychiatric condition and the specific minor cannabinoids under investigation.
Furthermore, to visualize the effect sizes of the most effective doses for the previously selected outcomes, we created a forest plot based on standardized mean difference (SMD) and 95% confidence intervals (95% CI). Hedge’s g was used as a standardized measure of effect size, and it was calculated based on sample sizes and the extracted group means and standard deviation.
All statistical analyses were performed using RStudio (R Core Team, 2024).
2.5. Risk of Bias (RoB)
The SYRCLE tool was used to assess the risk of bias (RoB) in preclinical studies (Hooijmans et al., 2014). This tool employs a set of signaling questions, addressing six different types of bias (selection, performance, detection, attrition, reporting, and “other sources of bias”), to categorize the articles into low, high, or unclear (moderate) risk of bias. Two additional items, pertaining to any process of blinding and any randomization mentioned in the study, were assessed, and reported separately. The “revised Cochrane risk-of-bias tool for randomized trials” (RoB 2) was used to evaluate RoB in the single randomized clinical study included (Sterne et al., 2019). RoB 2 encompasses questions related to five domains, including randomization process, intervention deviation, missing outcome data, outcome measurement, and result reporting. The RoB assessment was independently performed by two reviewers (GC, MV). Any disagreements were resolved through discussion, and when consensus could not be reached, reviewers (AB, LG) were consulted.
3. Results
The search strategy retrieved a total of 1612 articles, which was reduced to 1308 after removing duplicates. Thirty-five publications underwent full-text screening, leading to the exclusion of thirteen other articles. One additional article was identified by screening the reference lists of relevant studies. This process yielded a total of 23 articles for inclusion in this systematic review (see Fig. 1).
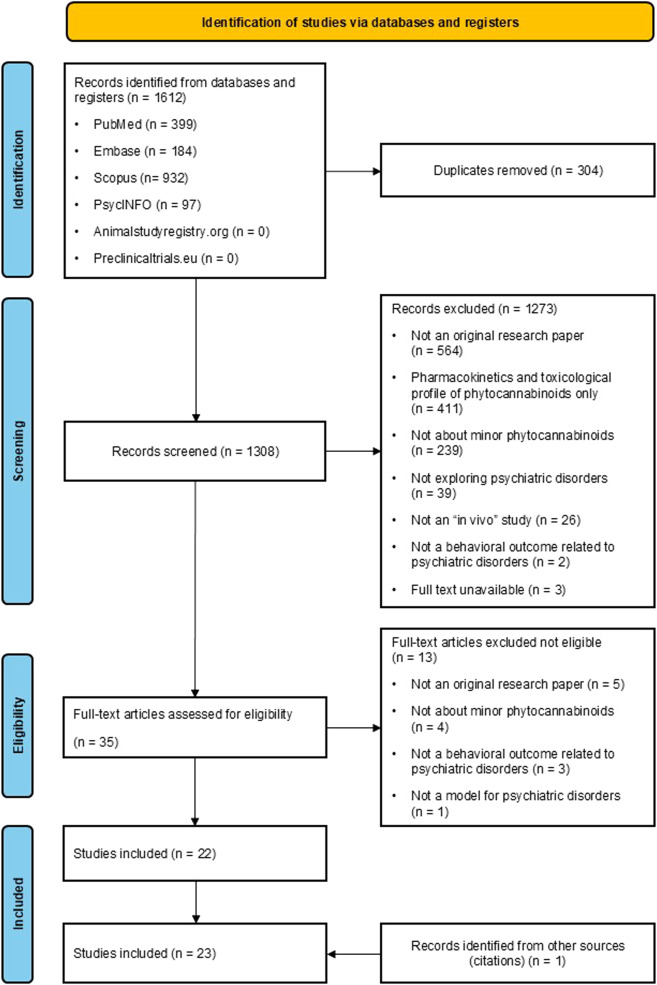
Fig. 1. PRISMA Flowchart.
3.1. Description of the included studies
Twenty-two articles explored the effects of minor cannabinoids on various proxies for psychiatric disorders in animal models. While some authors used specific disorder terms to refer to proxies for the conditions under study, we organized the retrieved studies according to the categories used in the latest edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM 5) (American Psychiatric Association, 2013). i) nine studies focused on substance-related and addictive disorders; ii) eight studies on anxiety disorders; iii) three studies on trauma- and stressor-related disorders; iv) three studies on depressive disorders; v) two studies on schizophrenia spectrum and other psychotic disorders; vi) one study on neurodevelopmental disorders.. Only one human study, which focused on psychotic disorders, met the inclusion criteria and was included in this systematic review.
Regarding the most studied minor cannabinoids: i) nine articles assessed the properties of CBDA; ii) five studies Δ8-THC; iii) four studies CBG; iv) four studies CBN; v) four studies Δ9-THCV; v) three studies CBDA synthetic analogue, cannabidiolic acid methyl ester (CBDA-ME or HU-580). While some authors have referred to CBDA-ME by its other name, HU-580, in this article we will refer to it only as CBDA-ME. Other assessed compounds included CBC (two studies), CBDV (two studies), 11-OH-Δ8-THC, a metabolite of Δ8-THC (two studies), Δ8-THCV (one study), and Δ9-THCA (one study). Some of the included articles examined the properties of multiple cannabinoids, compared them to one another and to the major cannabinoids Δ9-THC and CBD. Details about the characteristics of the included articles can be found in Table 1. Effect sizes for the most effective doses of the compounds under study for the previously selected outcomes are presented in the forest plot (Fig. 3). Three studies did not report information on sample size and/or error measures and thus were excluded from the forest plot (Chesher et al., 1985; Rock et al., 2014; Yamaguchi et al., 2001 ).











